Low-Dose CT for Lung Cancer Screening - CAM 391
GENERAL INFORMATION
It is an expectation that all patients receive care/services from a licensed clinician. All appropriate supporting documentation, including recent pertinent office visit notes, laboratory data, and results of any special testing must be provided. If applicable: All prior relevant imaging results and the reason that alternative imaging cannot be performed must be included in the documentation submitted.
Where a specific clinical indication is not directly addressed in this guideline, medical necessity determination will be made based on widely accepted standard of care criteria. These criteria are supported by evidence-based or peer-reviewed sources such as medical literature, societal guidelines and state/national recommendations.
Purpose
Low Dose Computed Tomography (LDCT) generates images of the lungs (chest) and is used to screen for and detect lung cancer in high-risk patients and/or patients with a history of lung cancer. This study uses low doses of radiation (100-120 kVp and 40-60 mAs) and is primarily used to evaluate the lung parenchyma. When evaluation of structures such as lymph nodes or the mediastinum is needed, a standard dose CT with IV contrast may be more appropriate.1
Policy
IINDICATIONS
For Annual Screening
The use of low-dose, non-contrast spiral (helical) multi-detector CT imaging as a screening technique for lung cancer is considered MEDICALLY NECESSARY ONLY when used to screen for lung cancer for certain high-risk, asymptomatic individuals, i.e., no acute lung-related symptoms, when ALL of the following criteria are met.
Screening should be discontinued once a person develops a health problem that limits the willingness or ability to have curative intent treatment.2,3
Group 1 — High Risk for Lung Cancer
- Individual is between 50 – 80 years of age*; AND
- There is at least a 20 pack-year history of cigarette** smoking
*May approve for individuals over the age limit if the individual is a candidate for and willing to undergo curative treatment upon diagnosis.
** Only personal cigarette smoking history as above places an individual at high risk; secondhand smoke exposure and other forms of smoking (such as pipe, cigar, marijuana,
vaping) do NOT factor into current recommendations for LDCT screening.
Group 2 — Personal History of Lung Cancer
Low Dose CT is indicated for surveillance of non-small cell lung cancer as follows:
Annually starting 3 years after the end of treatment if stage I – II and no history of radiation
Annually starting 6 years after end of treatment if EITHER stage I – II with history of radiation OR stage III or IV
NOTE: While on treatment, and for the first 2 – 3 years after completion of treatment, surveillance is with chest CT rather than LDCT. When radiation was used for treatment,
chest CT is needed for longer (5 years) before LDCT is appropriate. LDCT is not used for surveillance of small cell lung cancer4 (see Chest CT CAM 750).
Nodule on initial LDCT (Follow-up low dose CT is approvable)2
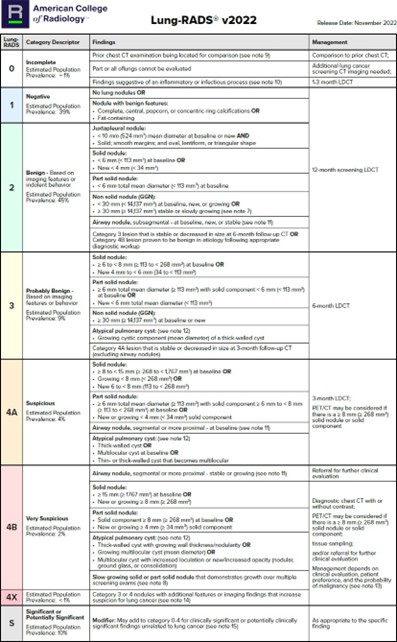
- Table 1 shows the follow-up interval at which LDCT can be approved to reduce radiation dose5
- If multiple nodules, the largest and type is used for decision.
Table 1: Lung-RADS®5
*This table is reproduced without alteration or edit in accordance with provisions in a Creative Commons License. The full document and license information can be found here: Lung Rads | American College of Radiology (acr.org)
Rationale
Smoking-related lung cancer is the leading cause of cancer deaths in both men and women in the United States. Treatment for most lung cancer is focused on surgery which is usually curative only when the tumors are very small. Screening for early lung cancer with sputum cytology and chest X-rays has not been successful in reducing deaths from lung cancer. However, in 2011, a large, prospective, multicenter trial was published that showed CT Chest screening identified early cancers better than other approaches and reduced the death rate from lung cancer. In 2014, the United States Preventive Service Task Force (USPSTF) recommended annual low-dose CT chest screening (CPT® code 71271) for people with current or recent past smoking histories.
The health effects of smoking (tobacco) products other than cigarettes is limited. More research is needed to explore the cancer risk from these products to guide cancer prevention efforts; therefore, cancer screening guidelines have not been developed for them. Currently, the screening guidelines apply only to cigarettes smoking.
All screening and follow-up chest CT scans to be performed at low dose (100 – 120 kVp and 40 – 60 mAs), unless evaluating mediastinal findings or lymph nodes, where standard dose CT with IV contrast may be more appropriate.4
OVERVIEW
Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery.
References
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer Version 3.2024. National Comprehensive Cancer Network®. 2024; 2024: Accessed: May 1, 2024. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Lung Cancer Screening Version 2.2024. National Comprehensive Cancer Network®. 2023; https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf.
- Wolf A, Oeffinger K, Shih T, Walter L, Church T et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2024; 74: 50 - 81. https://doi.org/10.3322/caac.21811.
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Small Cell Lung Cancer Version 2.2024. National Comprehensive Cancer Network®. 2024; Accessed: May 1, 2024. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf.
- ACR. American College of Radiology Lung-RADS System. American College of Radiology. 2022; Accessed: May 2024. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads
Coding Section
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2023 Forward
| 09/01/2025 | Annual review, no change to policy intent. |
| 09/24/2024 | Annual review, no change to policy intent. Updating references and Lung Rads table, policy reformatted for clarity and consistency. |
| 10/24/2023 |
New Policy |